research use only
Eflornithine (DFMO) Hydrochloride Hydrate Ornithine Decarboxylase Inhibitor
Cat.No.S4582
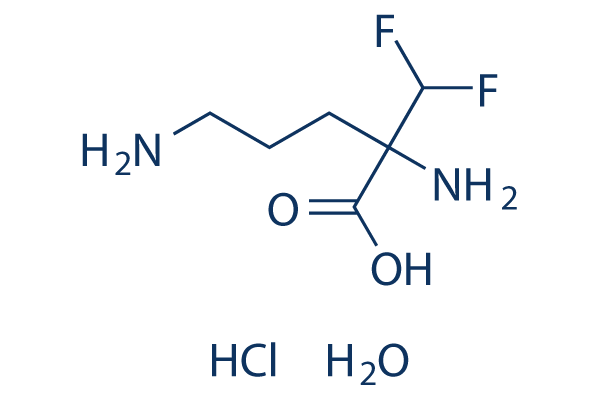
Chemical Structure
Molecular Weight: 236.64
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Decarboxylase Inhibitors | Benserazide HCl Ivospemin Maleic acid TES-1025 4-Bromo-3-hydroxybenzoic acid Chelidamic acid hydrate |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| J774.1 | Antitrypanosomal assay | 48 hrs | Antitrypanosomal activity against Trypanosoma brucei brucei TC221 infected in mouse J774.1 cells after 48 hrs by alamar blue assay, IC50 = 22.9 μM. | 22376072 | ||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
Water : 47 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 236.64 | Formula | C6H12F2N2O2.HCl.H2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 96020-91-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Difluoromethylornithine hydrochloride hydrate, MDL-71782 hydrochloride hydrate, RMI-71782 hydrochloride hydrate, α-difluoromethylornithine hydrochloride hydrate | Smiles | C(CC(C(F)F)(C(=O)O)N)CN.O.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Orn decarboxylase
|
|---|---|
| In vitro |
When cultured cells are treated with α-difluoromethyl-Orn, an inhibitor of polyamine biosynthesis, production of hydrogen peroxide is suppressed and programmed cell death did not occur. |
| In vivo |
Eflornithine alters changes in vascular responsiveness associated with coarctation hypertension |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | c-Myc / α-tubulin |
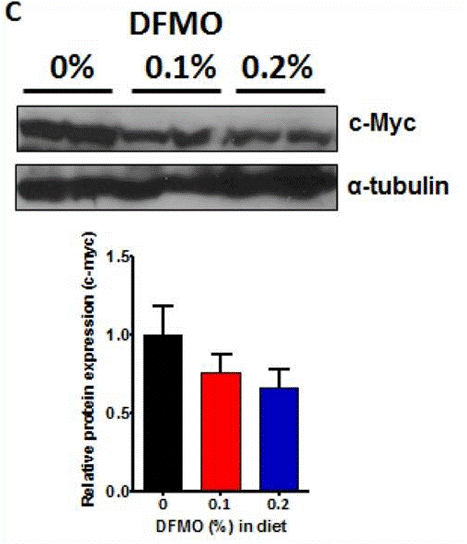
|
25248858 |
| Growth inhibition assay | 2T1 cells |
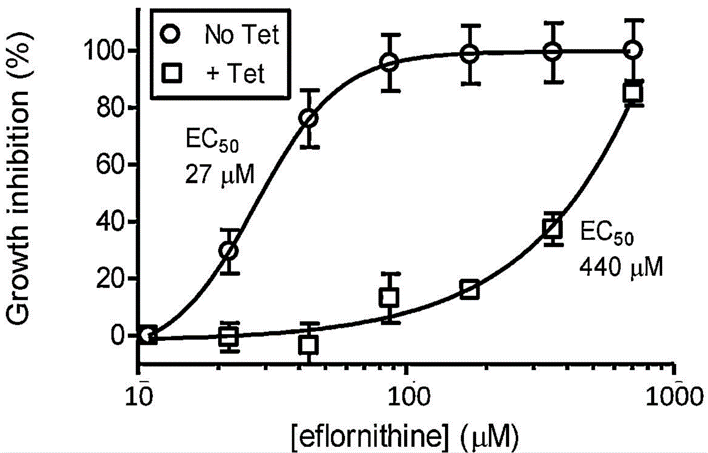
|
21093499 |
| IHC | H&E staining of pancreas p21 / PCNA |
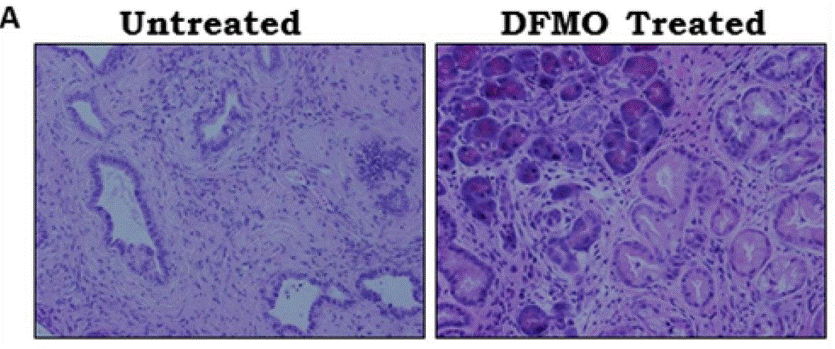
|
25248858 |
| IHC/IF | ODC expression in pancreatic tumors Cav-1 / β-catenin |
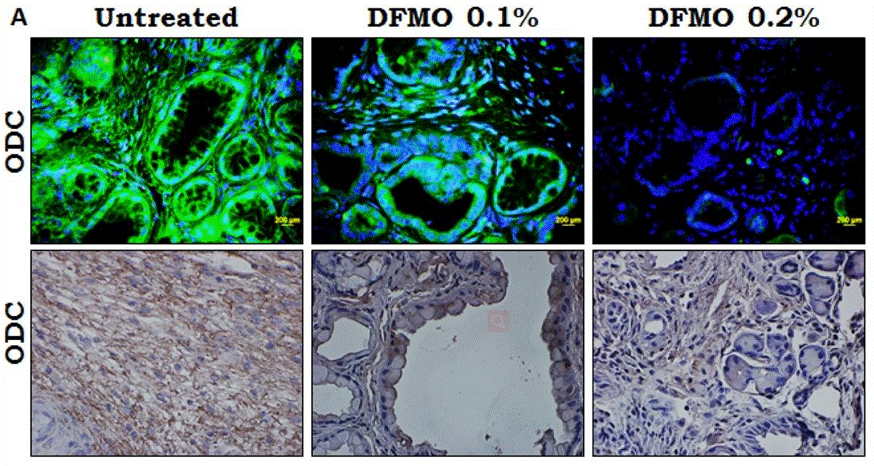
|
25248858 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05594563 | Recruiting | Type 1 Diabetes |
Emily K. Sims|Juvenile Diabetes Research Foundation|Cancer Prevention Pharmaceuticals Inc.|Indiana University |
March 14 2023 | Phase 2 |
| NCT05500508 | Recruiting | Cancer|Solid Tumor|Solid Carcinoma|Advanced Cancer|DIPG Brain Tumor|Ovary Cancer|Breast Cancer|Papillary Thyroid Cancer|Head and Neck Cancer|Gastric Cancer|Nsclc|Mesotheliomas Pleural|Mesothelioma Peritoneum|Esophageal Cancer|Diffuse Midline Glioma H3 K27M-Mutant|Endometrial Cancer|Cervical Cancer|Melanoma|Colorectal Cancer|Glioma Malignant |
Aminex Therapeutics Inc. |
November 29 2022 | Phase 1|Phase 2 |
| NCT04696029 | Recruiting | Medulloblastoma |
Giselle Sholler|Milton S. Hershey Medical Center |
March 29 2021 | Phase 2 |
| NCT03536728 | Completed | Cancer|Solid Tumor|Solid Carcinoma|Advanced Cancer |
Aminex Therapeutics Inc. |
June 12 2018 | Phase 1 |
| NCT02636569 | Active not recruiting | Non-melanoma Skin Cancer |
University of Alabama at Birmingham |
October 25 2017 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































